Paxlovid Wiki | Wznzawcpow3wlm
The SARS-COV-2 genome has several genes or ORFs. É um inibidor covalente ligando-se diretamente ao resíduo de cisteína catalítica Cys145 da enzima.
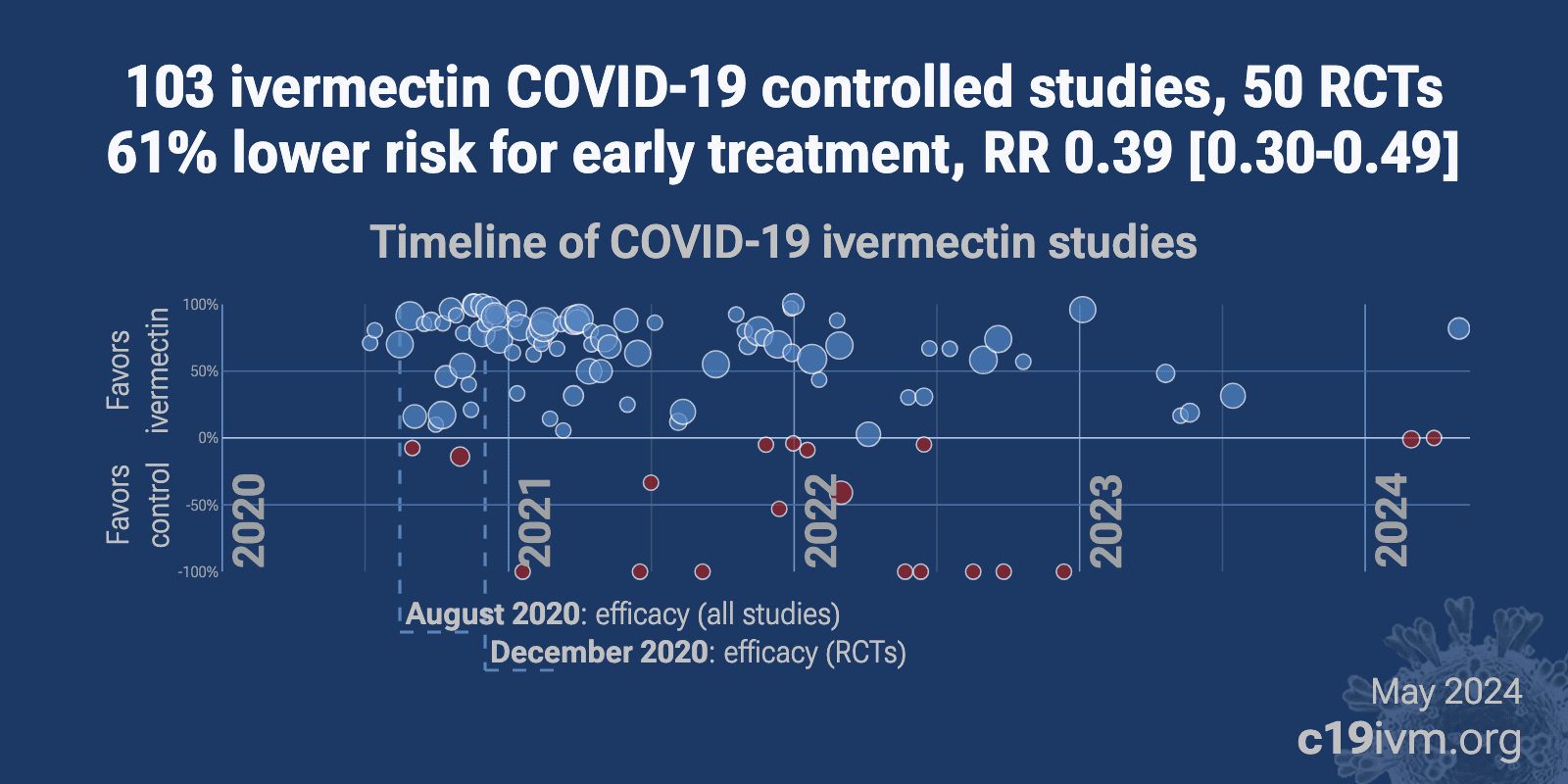
Ivermectin For Covid 19 Real Time Analysis Of All 128 Studies
This trademark was filed to UKIPO on Sunday May 9 2021.

Paxlovid wiki. According to a Pfizer study when given within five days of the onset of symptoms an antiviral therapy called Paxlovid prevented almost 90 percent of deaths from COVID-19 compared to placebo. Those taking the drug had an 89 reduction in their combined rate of hospitalisation or death after a month compared with patients taking a placebo. Pfizer has announced that its upcoming COVID drug Paxlovid seems to reduce the risk of hospitalization and death based on preliminary analysis of an ongoing study of.
Sanitary preparations for medical purposes. Dar es Salaam. Here a brief thread what this impressive new medicine does.
11Pfizer Says Its Antiviral Pill Paxlovid Cuts Rates Of Hospitalisation Death Risk of. Wonderful news below on Pfizers PAXLOVID pill. The PAXLOVID trademark covers Pharmaceutical preparations.
The antiviral pill is the second of. Paxlovid is a combination of an antiviral drug with ritonavir a drug usually used to treat HIVAids. Pfizer announced on Friday that its pill to treat Covid-19 had been found in a key clinical trial to be highly effective at preventing severe illness among at-risk people who received the drug soon after they exhibited symptoms.
Pfizers phase 23 trial randomized non-hospitalized adult COVID-19 patients who were at high risk of progressing to severe illness to receive placebo or Paxlovid a combination of the protease inhibitors PF-07321332 and ritonavir. The PAXLOVID is under the trademark classification. When given within five days of the onset of symptoms the antiviral therapy called Paxlovid prevented almost 90 of deaths from COVID-19 Thread starter Chriza28 Start date Today at 700 AM.
346296678 also pol wtf just pay for insurance to pay for these drug companies retarded high bills. The new drug developed by Pfizer offers the ability to virtually put an end to death from COVID-19. Kampuni ya kutengeneza dawa ya Pfizer Inc imetangaza matokeo ya majaribio ya vidonge dhidi ya virusi vya Corona Uviko-19 ambapo imethibitisha kuwa vidonge hivyo vinaweza kupunguza makali ya ugonjwa huo ikiwemo kumuepusha mtu kulazwa hospitalini au kifo.
The PAXLOVID trademark covers Pharmaceutical preparations. 1Está em estudos de fase 3 para o tratamento de covid-19 em combinação com ritonavir e em novembro de 2021 a Pfizer anunciou que em testes iniciais ele. This trademark was filed to EUIPO on Friday May 7 2021.
The pharmaceutical giant was quick to point out that their pill was developed with the specific goal of fighting Covid-19 unlike the Merck medication which was. April 13 2021 Pharmaceutical preparations for the treatment of autoimmune cardiovascular central nervous system endocrine gastrointestinal. If permitted or licensed Paxlovid can be the primary oral antiviral of its sort a particularly designed SARS.
Pfizer says antiviral pill Paxlovid 89 effective against COVID-19. Hot on the tail of Merck winning approval for use in the UK for its anti-viral Molnupiravir pill treatment Pfizer announced yesterday that clinical trials show their new drug Paxlovid to be highly effective in treating Covid-19. PF-07321332 - противірусний препарат розроблений компанією Pfizer перорально активний інгібітор протеази 3CL proЦе ковалентний інгібітор ферменту що звязується безпосередньо з каталітично-активним залишком цистеїну Cys145.
Ethiopias internal crisis has worsened significantly in recent days as rebel groups band together and the government declares a state-of-emergency. Want to learn how to make a map. Sanitary preparations for medical purposes.
PAXLOVID é um medicamento antiviral desenvolvido pela Pfizer que atua como um inibidor da protease 3CL. The latest data on paxlovid come from an interim analysis of 775 adults enrolled on the study. In another game changer against COVID-19 pandemic Pfizer Inc said its antiviral pill Paxlovid is 89 effective in stopping.
PAXLOVID is a united kingdom trademark and brand of Pfizer Inc New York 10017 UNITED STATES. Paxlovid - Pfizers Newly Developed Covid-Treating Pill That Cuts Hospitalizations and Deaths by 89 - The drug binds to an enzyme called a protease to stop the virus from replicating itself. The efficacy analysis is based on 1219 patients.
Custom Maps Pavlov Shack. Pfizer plans now to submit the info of the research to the US Meals and Drug Administration for Emergency Use Authorization EUA as quickly as doable. The PAXLOVID is under the trademark classification.
PAXLOVID is a trademark and brand of PFIZER INC New York New York 10017-5755 UNITED STATES. One noteworthy feature is the newness of this drug.
Pfizer One Of The World S Premier Biopharmaceutical Companies